This article is for informational purposes only and does not constitute medical advice. Always consult with a qualified healthcare provider before considering any peptide therapy.
BPC-157 nasal spray represents an unstudied delivery route for a peptide that already lacks FDA approval. While some users prefer nasal sprays for convenience, no clinical data confirms the safety or efficacy of this method.
Quick Takeaways
- BPC-157 nasal spray has never been studied in published human research, making its safety and effectiveness completely unknown
- Bioavailability through the nose remains questionable, with no data on how much peptide actually enters the bloodstream
- Commercial nasal spray products lack quality oversight, with studies showing 30% contain incorrect sequences and 65% exceed endotoxin safety limits
- Injectable and oral BPC-157 have at least minimal human data, making nasal administration the least evidence-based option on the market
What Is BPC-157 Nasal Spray?
BPC-157 nasal spray delivers the synthetic peptide BPC-157 through nasal mucosa rather than via injection or oral consumption. The peptide itself consists of 15 amino acids derived from a protective protein found naturally in gastric juice.
Online vendors market nasal spray formulations as “research chemicals” to sidestep FDA restrictions. These products typically contain BPC-157 dissolved in liquid with preservatives, packaged in standard nasal spray bottles.
The nasal route theoretically bypasses digestive enzymes that break down peptides when swallowed. Whether this translates to meaningful absorption for BPC-157, thought, remains unknown.
The Theoretical Appeal of Nasal Peptide Delivery
Intranasal administration offers conceptual advantages for peptide delivery based on nasal cavity anatomy. The nasal mucosa contains rich capillary networks that allow direct entry into systemic circulation, avoiding first-pass liver metabolism. This means the peptide is not immediately broken down by liver enzymes before reaching circulation, allowing a higher proportion of the active compound to remain intact and biologically available.
The olfactory region provides a potential pathway straight to the brain, bypassing the blood-brain barrier entirely. This nose-to-brain route could theoretically deliver peptides to neural tissues at lower doses than systemic administration requires.
Other therapeutic peptides have shown effects through nasal delivery. Insulin administered nasally improved cognition and cortical blood flow in human trials. Oxytocin nasal spray demonstrated effects on social behavior and anxiety in clinical studies.
Why Nasal Delivery Works for Some Peptides
Peptides face harsh conditions in the digestive tract where stomach acid and enzymes rapidly degrade them before absorption. Nasal mucosa avoids this proteolytic environment, preserving peptide structure during absorption.
The nasal cavity offers approximately 150 square centimeters of absorptive surface with high vascularization. Medications absorbed here enter circulation within minutes, similar to injection speed. As discussed in Springer Nature Link, the nasal epithelium is fairly specialized at keeping larger things out such that it is still relatively poor at absorbing peptides and possesses many digestive enzymes
Nasal delivery also eliminates injection-related complications like abscess formation, local irritation, or infection from poor sterile technique. This non-invasive approach appeals to individuals uncomfortable with needles.
Third-Party Tested, 99% Purity
Order lab-verified peptides from our top recommended vendor.

The Evidence Gap: What We Don’t Know About BPC-157 Nasal Spray
No peer-reviewed study has examined BPC-157 administered through the nose. The scientific literature on BPC-157 includes over 30 preclinical studies examining injection-based routes (intramuscular, intravenous, intraperitoneal, and oral), but nasal administration has not been systematically studied in published research.
This absence of data is notable given the known challenges of intranasal peptide delivery. A comprehensive 2022 review on nose-to-brain peptide delivery identified several barriers that would apply to BPC-157:
- Most successful intranasal peptide therapies required specialized delivery devices, not standard nasal sprays
- The olfactory region, where direct brain access occurs, comprises just 3-5% of total nasal cavity surface area and sits in the upper nasal passages where conventional sprays struggle to reach
- Metabolic enzymes in the olfactory mucosa can rapidly degrade peptides before they reach the brain
- The small surface area limits the dose that can be effectively absorbed
Compare this to injectable BPC-157, which has at least minimal human data. A pilot intravenous study in two healthy adults administered 10 and 20 milligrams of BPC-157 reported no observed adverse effects and no changes in cardiac, hepatic, renal, or metabolic markers. A knee pain case series reported improvements in 11 of 12 patients receiving intra-articular injection.
Unknown Pharmacokinetics
BPC-157 is rapidly cleared from circulation with a half-life under 30 minutes following injection. The peptide quickly breaks down into small fragments and eventually individual amino acids that enter normal metabolism.
Whether nasal absorption subjects BPC-157 to the same rapid degradation remains unknown. If the peptide enters systemic circulation through nasal mucosa, it would face identical enzymatic breakdown. The primary theoretical benefit would be nose-to-brain delivery that reaches neural tissues before systemic degradation occurs.
BPC-157 administered via injection has been detected in urine for up to four days using mass spectrometry, even though circulating levels of the intact peptide clear rapidly from plasma. This signal likely reflects the presence of identifiable BPC-157 peptide fragments rather than intact drug, as the compound is enzymatically cleaved after administration. Intranasal delivery has not been evaluated with pharmacokinetic or urinary detection studies, so persistence following nasal use remains unknown.
Mechanisms: How Nasal BPC-157 Might Work
BPC-157 activates signaling pathways involved in tissue repair, vascular integrity, and inflammatory control. In preclinical models, the peptide engages growth hormone–related signaling and downstream pathways including FAK–paxillin, JAK–STAT, and VEGFR2–Akt–eNOS, all of which play established roles in wound healing and endothelial function.
Activation of these pathways supports new blood vessel formation through vascular endothelial growth factor (VEGF), improves fibroblast migration and collagen organization during tissue repair, and suppresses pro-inflammatory cytokines such as TNF-α and IL-6. Together, these effects help restore damaged tissue structure while limiting excessive inflammatory signaling that can delay healing. Separately, animal studies suggest BPC-157 may influence dopamine and serotonin activity, which has been proposed as a mechanism for its reported effects on stress response and
If nasal delivery successfully transports BPC-157 through olfactory pathways to brain tissue, these neurotransmitter effects might occur at lower systemic doses. However, achieving meaningful olfactory delivery requires overcoming substantial anatomical barriers that remain poorly understood even for peptides specifically engineered for nasal use.
The Olfactory Pathway Challenge
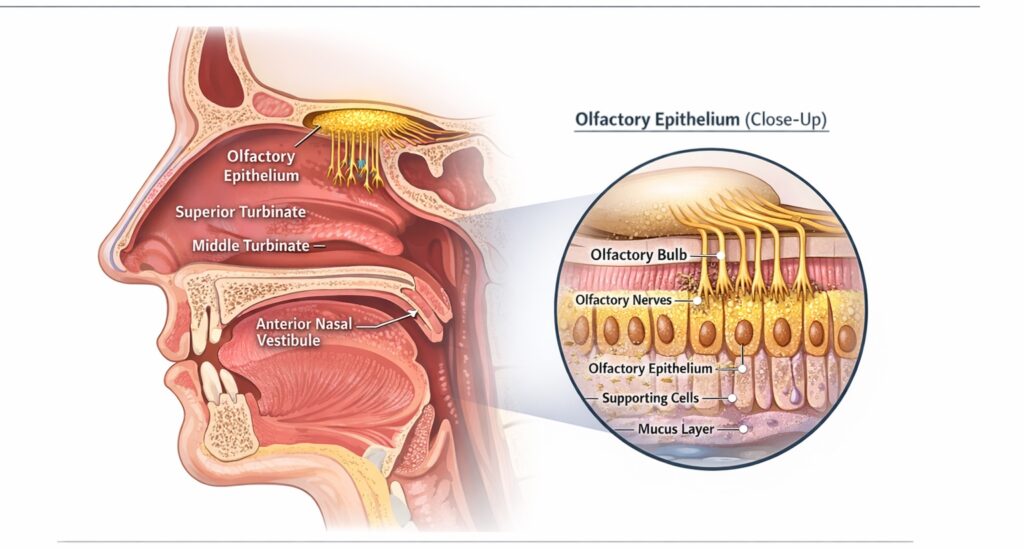
The olfactory epithelium occupies only a small, superior region of the nasal cavity and represents a limited fraction of total nasal surface area (Figure 1). This region sits above the inferior and middle turbinates, away from the primary airflow path during standard nasal spray administration.
Conventional liquid nasal sprays deposit most of their dose in the anterior nasal vestibule, where mucosal absorption is limited (Figure 2). Although approximately 95% of a sprayed dose enters the nasal cavity, only about 30% reaches deeper regions associated with improved absorption, with minimal exposure to the olfactory epithelium. As a result, delivery to olfactory tissue remains inefficient when using standard spray bottles.
Formulation and device design further constrain delivery. Dry powder nasal formulations demonstrate improved stability and, in some cases, enhanced bioavailability compared with liquid sprays, while specialized delivery devices increase deposition in superior nasal regions (Figure 3). Despite these advantages, commercially available BPC-157 nasal products rely on basic liquid spray mechanisms, limiting their ability to access olfactory mucosa in a consistent or measurable manner.
Figure 1. Anatomical Location of the Olfactory Epithelium Within the Nasal Cavity
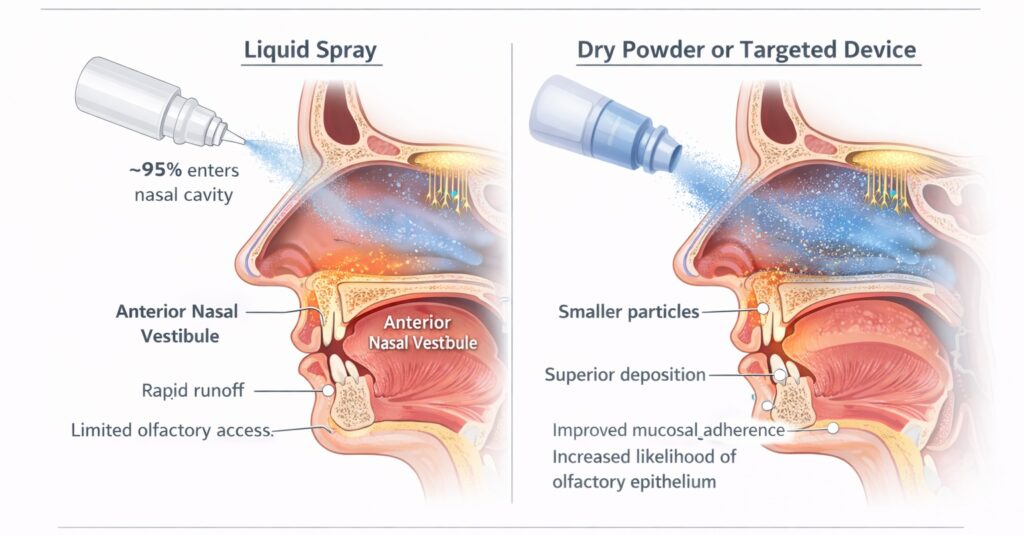
Figure 2. Deposition Pattern of Standard Liquid Nasal Sprays
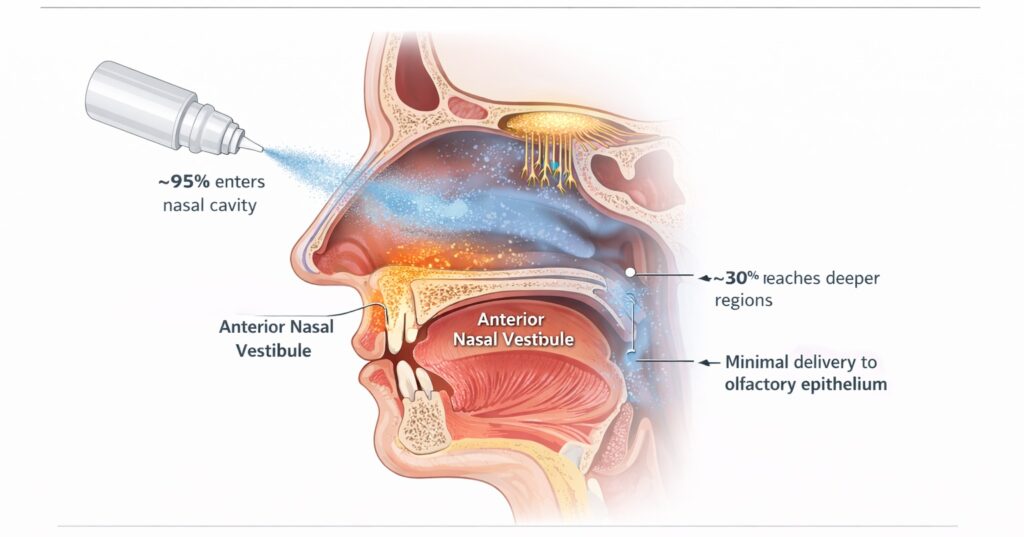
Figure 3. Impact of Formulation and Device Design on Olfactory Delivery
Reported Benefits of BPC-157 Nasal Spray: Anecdotal Claims Without Validation
Online forums contain numerous subjective reports from individuals claiming benefits from BPC-157 nasal spray. Users describe improved healing from injuries, reduced pain and inflammation, and enhanced cognitive function, often reporting effects within days.
These anecdotal reports cannot be distinguished from placebo responses without controlled trials. Placebo effects for musculoskeletal pain average approximately 30% in clinical trials, potentially approaching 50% when individuals pay out-of-pocket for treatments they believe should work.
Many users simultaneously make other lifestyle changes—modifying exercise patterns, beginning physical therapy, improving sleep—that could independently drive symptom improvement. Musculoskeletal injuries often naturally improve over time regardless of specific interventions.
Dosing Recommendations: Speculation Without Foundation
Commercial vendors recommend wildly inconsistent doses reflecting guesswork rather than evidence. Some suggest single sprays once or twice daily containing unstated BPC-157 amounts. Others recommend multi-spray protocols with total daily doses ranging from 100 micrograms to several milligrams.
Injectable BPC-157 protocols typically use 250 to 500 micrograms daily, with some approaches reaching 1,000 micrograms daily. Oral dosing recommendations range from 200 to 500 micrograms daily, though oral absorption is substantially poorer than that achieved by injection.
If nasal bioavailability is lower than injection—a reasonable expectation based on current evidence—achieving equivalent systemic exposure might require substantially higher intranasal amounts. Whether the nasal mucosa can tolerate such quantities of peptide without irritation or local toxicity remains unknown.
Practical Dosing Considerations
The concentration of BPC-157 in commercial nasal spray formulations varies between vendors and often is not clearly stated on labels. Users cannot reliably calculate actual doses administered per spray.
Online recommendations typically suggest 4- to 8-week cycles followed by breaks, based on analogy to injectable protocols. This reasoning lacks any evidentiary foundation specific to nasal administration.
No dose-ranging studies have examined what plasma concentrations can be achieved by different nasal doses of BPC-157. Standard approaches to deriving human doses from animal studies depend on understanding pharmacokinetic parameters that simply do not exist for nasal BPC-157.
BPC-157 Delivery Route Comparison
| Route | Human Data | Bioavailability | Convenience | Quality Control | Cost |
|---|---|---|---|---|---|
| Nasal Spray | None | Unknown (likely low) | High | None (unregulated) | Moderate |
| Subcutaneous Injection | Minimal case reports | High | Moderate | Variable | Low-Moderate |
| Oral Capsules | None | Low (gastric degradation) | High | Variable | Moderate |
| Intravenous | One pilot study (2 subjects) | 100% | Low (clinical setting) | Variable | High |
Safety and Contraindications
BPC-157 nasal spray carries multiple uncharacterized safety risks stemming from the complete absence of human safety data. No clinical trials, toxicology studies, or formal pharmacokinetic investigations have examined intranasal BPC-157 in any population.
Potential risks include local mucosal irritation, allergic reactions, and systemic immune responses triggered by endotoxins or peptide impurities. The nasal epithelium’s rich vascularization means contamination entering the nasal cavity has direct access to bloodstream circulation.
Product Quality Concerns
BPC-157 is classified as a Category 2 bulk drug substance, prohibiting legal compounding by commercial pharmacies. Products sold as nasal sprays come from unregulated manufacturers without Good Manufacturing Practice oversight.
Studies examining online peptide products found 30% contained incorrect amino acid sequences. Additionally, 65% had endotoxin levels above safety thresholds, which could trigger significant inflammation when absorbed through nasal mucosa.
There is no assurance of sterility, pyrogenicity control, or accurate dosing in commercial BPC-157 nasal spray formulations. Users cannot verify whether bottle contents represent active BPC-157 or degraded fragments of uncertain bioactivity.
Who Should Avoid BPC-157 Nasal Spray
Individuals with active cancer or cancer history should exercise particular caution. BPC-157 stimulates angiogenesis through VEGF pathways, raising theoretical concerns about tumor growth promotion, though no such effects have been demonstrated in humans.
Pregnant or breastfeeding women should avoid BPC-157 entirely given the absence of safety data in these populations. Similarly, individuals under 18 lack any safety or efficacy data supporting the use of this peptide.
Those taking anticoagulant medications should consult healthcare providers before considering BPC-157, as the peptide may influence clotting factors based on animal research. No studies of interactions between BPC-157 and other medications exist.
Athletes subject to anti-doping testing should avoid BPC-157 completely. The World Anti-Doping Agency prohibits BPC-157 under the S0 category of non-approved substances, and the peptide remains detectable in urine for up to four days.
The Regulatory Reality
The FDA has not approved BPC-157 for any therapeutic indication. Agency officials cite “risk for immunogenicity, peptide-related impurities, and limited safety-related information” as reasons for restricting commercial compounding.
Vendors market BPC-157 nasal spray as “research chemicals” labeled “not for human consumption” to evade FDA oversight. This legal fiction allows sales while products are simultaneously marketed with implicit or explicit therapeutic claims.
Consumers purchasing these products participate in uncontrolled human experimentation without institutional review board oversight, informed consent processes, or adverse event monitoring systems.
Frequently Asked Questions
Does BPC-157 nasal spray work as well as injections?
No published research compares nasal spray to injectable BPC-157, making direct effectiveness comparisons impossible. Injectable forms have at a limited number of human case reports showing potential benefits, whereas there is no clinical data supporting nasal spray formulations. The questionable bioavailability associated with nasal administration suggests injectable routes likely deliver more peptide to target tissues.
How long does it take for BPC-157 nasal spray to work?
Anecdotal reports claim effects within days, but these subjective experiences cannot be separated from placebo responses without controlled trials. Injectable BPC-157 in animal studies showed effects on tissue healing within 7-14 days. Whether nasal administration produces similar timelines remains unknown given the lack of absorption data.
Can I use BPC-157 nasal spray for brain fog or cognitive issues?
BPC-157 shows effects on dopamine and serotonin pathways in rodent models, theoretically supporting cognitive applications. However, no human studies have examined BPC-157 for cognitive symptoms through any route. The nose-to-brain pathway that might deliver BPC-157 directly to neural tissue remains theoretical for this peptide specifically.
Is BPC-157 nasal spray safer than injectable forms?
Nasal spray avoids injection-related risks like infection from poor sterile technique or injection-site reactions. However, nasal formulations carry their own risks including mucosal irritation, contamination-related immune responses, and unknown long-term effects on nasal tissue. The lack of human safety data makes meaningful safety comparisons impossible.
The Bottom Line: Weighing an Unstudied Option
BPC-157 nasal spray combines an unapproved peptide with an unstudied delivery route, creating a product that exists entirely outside established medical evidence. While the theoretical basis for nasal peptide delivery is scientifically sound for some compounds, BPC-157 specifically has never been evaluated through this route in any published research.
The complete absence of data on bioavailability, appropriate dosing, safety, or efficacy makes nasal BPC-157 the least evidence-based option among available delivery routes. Injectable and oral forms have at least minimal human reports, while nasal spray has none.
Individuals considering BPC-157 nasal spray should recognize they are participating in uncontrolled self-experimentation with products lacking quality assurance. Well-designed clinical trials comparing nasal BPC-157 to placebo and established treatments would be necessary to determine whether this delivery route offers any legitimate advantages.
References
- Nose-to-Brain Delivery of Therapeutic Peptides as Nasal Aerosols. Pharmaceutics. 2022;14(9):1870. doi:10.3390/pharmaceutics14091870. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9502087/
- Overview of intranasally delivered peptides: key considerations for pharmaceutical development. Expert Opin Drug Deliv. 2018;15(10):991-1005. doi:10.1080/17425247.2018.1517742. Available from: https://pubmed.ncbi.nlm.nih.gov/30173579/
- What is the efficacy of intranasal BPC-157 (Body Protection Compound 157)? Published April 8, 2025. Accessed December 29, 2025. https://droracle.ai/articles/66566/what-is-the-efficacy-of-intranasal-bpc-157-body-protection-compound-157
- Safety of intravenous infusion of BPC157 in humans: a pilot study. Altern Ther Health Med. 2025;31(5):20-24. Accessed December 29, 2025. https://pubmed.ncbi.nlm.nih.gov/40131143/
- Pharmacokinetics, distribution, metabolism, and excretion of body protective compound-157 in rats. Front Pharmacol. 2022;13:1043062. Accessed December 29, 2025. https://pmc.ncbi.nlm.nih.gov/articles/PMC9794587/
- Detection and in vitro metabolism of the confiscated peptides BPC-157 and PEG-MGF. Drug Test Anal. 2017;9(7):996-1006. Accessed December 29, 2025. https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/dta.2152
- New Regen Ortho. The hidden risks of BPC‑157: what patients need to know about contamination and safety. Published August 5, 2025. Accessed December 29, 2025. https://newregenortho.com/the-hidden-risks-of-bpc-157-what-patients-need-to-know-about-contamination-and-safety/
- US Food and Drug Administration. Certain bulk drug substances that may present significant safety risks for use in compounding. Updated August 6, 2025. Accessed December 29, 2025. https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks
- World Anti-Doping Agency. The prohibited list. Updated October 2, 2025. Accessed December 29, 2025. https://www.wada-ama.org/en/prohibited-list
- Emerging use of BPC-157 in orthopaedic sports medicine. HSS J. Published online July 30, 2025. Accessed December 29, 2025. https://pmc.ncbi.nlm.nih.gov/articles/PMC12313605/
