[Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with a qualified healthcare provider before starting any peptide therapy.]
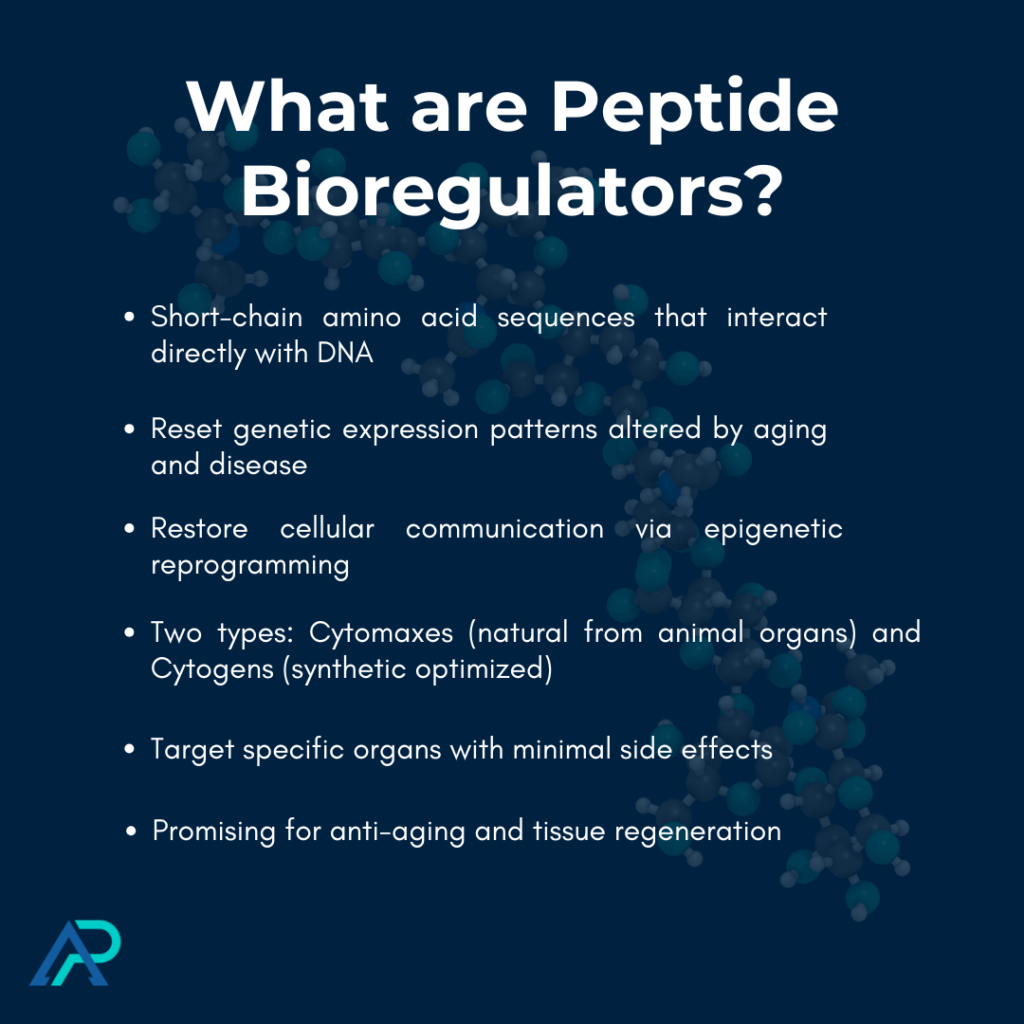
Peptide bioregulators are a fascinating frontier where molecular biology meets regenerative medicine. These short-chain amino acid sequences—typically just two to seven amino acid residues long—work as sophisticated biological messengers that interact directly with our DNA.
Unlike regular peptides that just stimulate existing pathways, bioregulators actually reset genetic expression patterns that get altered by aging or disease.
These compounds came from Cold War-era Soviet research led by Professor Vladimir Khavinson. They were first developed to tackle accelerated aging seen in military personnel exposed to extreme conditions.
What started as classified research has grown into a validated field of geroscience with forty years of clinical use.
What makes peptide bioregulators special is their ability to modify epigenetics—they don’t just temporarily boost function but restore optimal cellular communication pathways that break down as we age.
This marks a shift from treating symptoms to addressing the root causes of age-related decline.
At AllAboutPeptides.com, we highlight both the groundbreaking science behind these compounds and their experimental status in Western medicine, staying true to our commitment to balanced education about these intriguing biological tools.
Key Takeaways
- Peptide bioregulators are short-chain amino acid sequences that interact directly with DNA, resetting genetic expression patterns altered by aging or disease rather than just stimulating existing pathways.
- There are two main types: Cytomaxes (natural tissue-specific peptides extracted from animal organs) and Cytogens (synthetic optimized analogs with enhanced bioavailability).
- These compounds demonstrate remarkable tissue selectivity, targeting specific organ systems with minimal side effects, and have shown promising results in anti-aging, tissue regeneration, and immune modulation applications.
- Despite decades of clinical use and a favorable safety profile, peptide bioregulators face regulatory challenges that have affected their mainstream adoption and accessibility.
The Science Behind Peptide Bioregulators
Peptide bioregulators work through a fascinating biological process that sets them apart from ordinary supplements or medications. Understanding this process helps explain their unique effects on aging and tissue regeneration.
Receptor Binding and Signal Amplification
When bioregulators enter your system, they bind to specific receptors on cell membranes—primarily G-protein coupled receptors and integrins. This binding initiates a phosphorylation cascade that activates intracellular messengers like cyclic AMP.
What’s remarkable is how this process amplifies the original signal. A tiny amount of peptide can create a significant cellular response, which is why nanomolar concentrations can produce measurable changes in gene expression.
Epigenetic Reprogramming
The real magic happens at the genetic level. These signals activate transcription factors that regulate three critical functions:
- Protein synthesis for cellular rebuilding
- DNA repair mechanisms
- Control of cellular senescence
Unlike conventional peptides, bioregulators exhibit histone acetyltransferase activity—they can actually remodel chromatin structure to expose previously silenced genes associated with youth and longevity.
For example, in lymphocytes from elderly donors, Ala-Glu-Asp-Pro (Cortagen) increased ribosomal gene activation by 27% while maintaining pericentromeric heterochromatin stability1. This selective deheterochromatinization reactivates silenced genes without destabilizing structural DNA elements, a critical factor in their anti-carcinogenic properties.
These mechanisms explain why bioregulators don’t just temporarily boost function but potentially restore more youthful cellular behavior. They address what researchers call the “information theory of aging”—the gradual loss of precise biological instructions that occurs over time.
Main Types of Peptide Bioregulators
Professor Khavinson’s pioneering research established two fundamental categories of peptide bioregulators, each with distinct origins and applications in therapeutic settings.
Cytomaxes: Natural Tissue-Specific Peptides
Cytomaxes are bioregulators extracted directly from animal organs that mimic human regulatory sequences. These naturally occurring compounds maintain the complex structural elements developed through evolution.
They work by targeting specific tissues with remarkable precision, helping maintain homeostasis despite age-related degenerative pressures.
For instance, retinal peptides derived from bovine eyes enhance photoreceptor function in humans, as demonstrated in pediatric epilepsy patients with optic atrophy2.
The structural homology between mammalian peptide sequences enables cross-species therapeutic effects without triggering immune responses, owing to their low molecular weight (<5 kDa).
Cytogens: Synthetic Optimized Analogs
Cytogens represent the next generation of bioregulators—synthetic analogs optimized for enhanced bioavailability and target affinity. These laboratory-created peptides maintain the core functional sequences while improving stability, absorption, and cellular penetration.
Cytogens employ truncated sequences (3-7 amino acids) synthesized from plant-derived amino acids. While achieving faster pharmacokinetic effects due to higher concentrations and shorter chain lengths, their duration of action remains limited to 1-2 months compared to natural peptides’ 2-3 month post-effect.
The synthetic Epithalon (Ala-Glu-Asp-Gly) exemplifies this category, demonstrating telomere elongation through telomerase activation in human trials3.
Organ-Specific Targeting
A defining characteristic of peptide bioregulators is their remarkable tissue selectivity. Different peptides target specific organ systems:
- Cardiovascular system: Vesugen promotes vascular repair4
- Nervous system: Cortexin supports neurological function5
- Immune system: Thymalin helps normalize immune responses6
- Geroprotection: Epithalamin reduces oxidative stress markers
- Musculoskeletal system: Sigumir targets bone tissue regeneration
This organ-specific tropism allows precise therapeutic applications without systemic side effects—one of the key advantages bioregulators hold over broader interventions.
Third-Party Tested, 99% Purity
Order lab-verified peptides from our top recommended vendor.

Therapeutic Applications of Peptide Bioregulators
Peptide bioregulators demonstrate versatility across multiple therapeutic domains, with decades of clinical data supporting their efficacy.
Anti-Aging and Longevity Enhancement
Clinical studies tracking elderly patients receiving regular courses of peptide bioregulators have documented significant improvements in key biomarkers associated with aging. These include enhanced telomerase activity, reduced inflammatory markers, and improved cognitive function7.
Long-term studies suggest these improvements correlate with meaningful reductions in all-cause mortality compared to control groups.
Tissue Regeneration
Different bioregulators show remarkable tissue-specific effects:
- Cardiovascular: Vascular peptides help improve heart function after myocardial injury through mechanisms including cardiomyocyte proliferation and enhanced angiogenesis.
- Neurological: Brain-specific peptides demonstrate neuroprotective effects in various degenerative conditions, with studies showing improvements in cognitive assessment scores through mechanisms including enhanced β-amyloid clearance.
- Musculoskeletal: Bone-targeting peptides promote increased mineral density in conditions like osteoporosis through improved calcium metabolism and bone tissue regeneration.
Metabolic and Immune Modulation
Pancreatic-derived peptides show insulin-sensitizing properties8 comparable to conventional treatments, while thymic peptides help normalize immune cell ratios in various immunological disorders9.
A key advantage of bioregulators is their bifunctional nature—simultaneously supporting tissue repair while moderating excessive inflammatory responses. This balanced approach helps address both the primary condition and its secondary complications, providing a more comprehensive therapeutic effect than many single-target interventions.
Administration and Dosing Considerations
Successful therapeutic outcomes with peptide bioregulators depend significantly on appropriate administration methods and dosing protocols. Research indicates specific approaches yield optimal results.
Peptide bioregulators are formulated as oral capsules, offering a convenient and consistent delivery method. Dosing recommendations typically follow established patterns based on clinical research:
- Standard protocols suggest 1-2 capsules (containing 10-20mg) daily with meals
- Individual response varies, sometimes necessitating personalized adjustments based on clinical outcomes
Long-term studies indicate that cyclical administration protocols help maintain efficacy:
- Standard protocols often recommend 30-60 day treatment courses
- Rest intervals of 4-6 months between courses prevent receptor desensitization
- This cycling approach better maintains cellular responsiveness compared to continuous administration
The timing and consistency of administration significantly impact results:
- Taking capsules with meals improves absorption and tolerability
- Consistent daily timing enhances cellular response patterns
- Effects from properly administered courses may persist for several months after completion
These administration principles help maximize the therapeutic potential of peptide bioregulators while supporting their practical integration into everyday wellness routines.
Safety Profile and Potential Side Effects
Clinical experience with peptide bioregulators suggests a favorable safety profile when used appropriately.
Studies spanning several decades indicate:
- Low incidence of reported adverse effects in clinical settings
- Natural metabolic processing through normal protein degradation pathways
- Absence of toxic accumulation in long-term administration studies
Prudent application includes:
- Consulting healthcare providers before beginning any peptide regimen
- Following recommended dosing and cycling protocols
- Avoiding use in pregnant or nursing women unless specifically advised
- Monitoring for individual sensitivity or response variations
Further research continues to evaluate long-term outcomes across diverse populations. As with any biologically active compound, individual responses may vary, and medical supervision is recommended, particularly for those with existing health conditions.
Regulatory Status and Accessibility
Peptide bioregulators occupy a complex position in the regulatory landscape. Despite accumulating evidence supporting their efficacy, these compounds face several challenges in mainstream adoption. Their status as naturally occurring substances makes them difficult to patent, limiting commercial research investment.
Additionally, they often fall into ambiguous classification categories between dietary supplements and biological therapeutics, creating inconsistent regulatory frameworks across different jurisdictions.
Recent regulatory changes, including the FDA’s 2023 reclassification of certain signaling peptides10, have affected availability of some synthetic analogs, though natural peptide bioregulators generally remain accessible as nutraceuticals.
These regulatory considerations continue to evolve as research expands and consumer interest grows, potentially shaping future access and application protocols.
The Place of Peptide Bioregulators in Modern Healthcare
Peptide bioregulators constitute a paradigm shift in preventive medicine, offering a unique confluence of genomic precision and systemic biocompatibility. Their capacity to reset age-related epigenetic changes while stimulating organ-specific regeneration positions them as indispensable tools in the geroscience arsenal.
Future integration with omics-based personalization platforms and targeted delivery technologies promises to unlock their full potential, potentially extending healthspan beyond current theoretical limits.
However, realizing this potential requires resolving regulatory ambiguities and conducting large-scale, multinational trials to cement their role in mainstream medical practice.
References
- https://pubmed.ncbi.nlm.nih.gov/37042594/ ↩︎
- https://www.semanticscholar.org/paper/Infrasound-phonophoresis-with-peptide-bioregulators-%D0%A1%D1%83%D1%85%D0%B0%D0%BD%D0%BE%D0%B2%D0%B0-%D0%A1%D0%B8%D0%B4%D0%BE%D1%80%D0%B5%D0%BD%D0%BA%D0%BE/bbc99e50c705f9d719c69fca4e058052c63b0e3b ↩︎
- https://pubmed.ncbi.nlm.nih.gov/37042594/ ↩︎
- https://jordantimes.com/news/business-club/vesugen-peptide-unraveling-its-potential-cellular-and-vascular-science ↩︎
- https://journals.eco-vector.com/RCF/article/view/456396 ↩︎
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8654498/ ↩︎
- https://pubmed.ncbi.nlm.nih.gov/24003726/ ↩︎
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3262724/ ↩︎
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6481824/ ↩︎
- https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks ↩︎
